INTRODUCTION
The process by which we convert water into steam and use the steam to turn a propulsion shaft encompasses the generation and expansion phases of the steam cycle. A study of the properties of water and steam at these critical phases is necessary to understand the steam cycle. This lesson defines terms associated with these properties and processes, and explains the use of steam tables to calculate the work and efficiency created by steam.
REFERENCES
(a) Elements of Applied Thermodynamics, Robert M. Johnson, et al.
(b) Principles of Naval Engineering NAVPERS 10788 series
(c) Introduction to Naval Engineering, Edward F. Gritzen.
INFORMATION
A. Basic Thermodynamic Terms
1. Enthalpy (h), measured in British thermal units per pound (mass), or BTU/lbm, represents the total energy content of steam. It expresses the internal energy and flow work, or the total potential energy and kinetic energy contained within a substance. The advantage of enthalpy is that we can express in one term all of the energy in a substance which is due to its pressure and temperature. Enthalpy values are used to represent the energy level of steam entering a turbine, a value useful for determining turbine efficiency. By superheating steam, we can add enthalpy to steam without raising the pressure of the steam. For example, steam at 620 psig and 850°F can do more work in a turbine than steam that is 620 psig and 650°F.
2. Entropy (s), measured in BTU/lbm-°R, represents the unavailability of energy (°R=Rankine temperature scale where 0°R = absolute zero and 460°R = 0°F). The second law of thermodynamics states that when heat is transferred from high temperature to low temperature regions, some of the heat will be rejected and not converted into mechanical work. Entropy is a measure of how much heat must be rejected to a lower temperature receiver at a given pressure and temperature.
a. A complex explanation of the mathematical significance of the definition of entropy is unnecessary. It is a term which attempts to describe the universe’s tendency to evenly distribute all mass and energy throughout space. Processes which produce entropy are possible and those which destroy entropy are impossible.
b. Bodies with a high temperature will, when brought in contact with a body of a lower temperature, always cause heat to transfer from the hot body to the cold body. This will lower the internal energy of the hot body and raise the internal energy of the cold body. This is the principle that guides the design and operation of all naval heat exchangers. For example, a main engine lube oil cooler directs hot lube oil over cool seawater piping, so that the hot lube oil will transfer some of its heat to the cooler seawater. If left together indefinitely, the property of entropy would cause the heat from the lube oil to be equally distributed between the oil and the water, so that both would have the same temperature.
c. Entropy would not be important except for the fact that the purpose of any engine is to collect, transfer, and use energy. Thus, in a steam plant for example, it is not possible to add energy to water, boil it and transmit the resulting high energy steam across the relatively cooler engineroom without some of that energy being lost. Some of this energy will always be lost through system conditions such as ineffective pipe lagging, piping leaks, and dirty or fouled tubes which retard heat transfer. Operators must constantly attempt to minimize the effects of these conditions to maximize plant efficiency and reduce fuel and water costs.
3. A working fluid is a substance which receives, transfers and transmits energy in a thermodynamic system. In most systems, the working substance is a fluid (liquid, vapor or gas). In a steam system, water is the working fluid.
4. Density (r), measured in lbm/ft, represents the mass of a substance per unit volume, or how tightly packed the molecules are. The more molecules packed in a given space, the more dense the material. The density of water in a given location of the boiler is critical to the steam generation process because relatively dense feedwater will naturally push a less dense steam/water mixture through the boiler generating tubes.
5. Specific volume (vSP), measured in ft3/lbm, represents the space occupied per unit mass of a substance. It is the mathematical inverse of density. Most engineering equipment is designed for size and strength taking into consideration the specific volume of the intended working fluid.
6. Specific weight (g), measured in lbf/ft3, represents the weight of a substance per unit volume. This is the density of a substance acted upon by gravity. The pressure of a fluid at the bottom of a storage tank is a direct function of the height of the fluid in the tank and the specific weight of the feedwater. This resultant pressure is an important shipboard consideration with respect to providing a minimum suction pressure for a pump below the tank to move the fluid through a system.
7. The state of a working fluid refers to the physical properties it possesses at a particular pressure, temperature and volume. If each of these are known with respect to a substance, the state of the substance is known. The substance can be a subcooled, saturated, or superheated solid, liquid, or gas. Many systems operate the working fluid with very specific temperature/pressure relationships. Water is subcooled in the condensate and feed phases of the steam cycle to allow it to be pumped, saturated in portions of the generation and feed phases for natural flow or for maintaining proper chemistry, and superheated in the expansion phase to extract maximum work from the steam to turn a propulsion turbine.
8. A thermodynamic process is any process which changes the state of the working fluid. These processes can be classified by the nature of the state change that takes place. Common types of thermodynamic processes include the following:
a. A reversible process is an ideal process where the working fluid returns to its original state by conducting the original process in the reverse direction. For a process to be reversible, it must be able to occur in precisely the reverse order. All energy that was transformed or distributed during the original process must be capable of being returned to its exact original form, amount and location. Reversible processes do not occur in real life.
b. An irreversible process is any process which is not reversible. All real life processes, such as the basic steam cycle, are irreversible.
c. An adiabatic process is a state change where there is no transfer of heat to or from the system during the process. Because heat transfer is relatively slow, any rapidly performed process can approach being adiabatic. Compression and expansion of working fluids are frequently achieved adiabatically with pumps and turbines.
d. An isothermal process is a state change in which no temperature change occurs. Note that heat transfer can occur without causing a change in temperature of the working fluid. In the DFT, auxiliary exhaust heats incoming condensate, then condenses to liquid and falls to the bottom of the tank. Throughout this process, the temperature of the auxiliary exhaust remains constant at 246-249°F.
e. An isobaric process is a state change in which the pressure of the working fluid is constant throughout the change. An isobaric state change occurs in the boiler superheater, as the heat of the exiting steam is increased without increasing its associated pressure.
9. A thermodynamic cycle is a recurring series of thermodynamic processes through which an effect is produced by the transformation or redistribution of energy. In other words, it is a series of processes repeated over and over again in the same order. Thermodynamic cycles contain five basic elements: (1) a working fluid, (2) an engine, (3) a heat source, (4) a heat receiver, and (5) a pump. All thermodynamic cycles may be classified as being open cycles or closed cycles.
a. A closed cycle is one in which the working fluid is reused. Steam plants and refrigeration cycles are closed cycles. In a steam plant, the water undergoes a series of processes that change the state of the water. Eventually the water returns to its original state and is ready to begin the cycle again.
b. An open cycle is one in which the working fluid is not reused. Open cycles typically use the atmosphere as a working fluid. An internal combustion engine represents a typical open cycle. Air is drawn into the engine, combusted in the cylinders, and exhausted back to the atmosphere. Fresh air is drawn into the engine to begin the cycle again.
B. Heat Addition and Temperature
1. When heat is added to a material, one of two things will occur: the material will change temperature or the material will change state. When a substance is below the temperature at a given pressure required to change state, the addition of sensible heat will raise the temperature of the substance. Sensible heat applied to a pot of water will raise its temperature until it boils. Once the substance reaches the necessary temperature at a given pressure to change state, the addition of latent heat causes the substance to change state. Adding latent heat to the boiling water does not get the water any hotter, but changes the liquid (water) into a gas (steam).
2. One can state that a certain amount of heat is required to raise the temperature of a substance 1°F. This energy is called the specific heat capacity. The specific heat capacity of a substance depends upon the volume and pressure of the material. For water, the specific heat capacity is 1 BTU/lbm-°F and remains constant. This means that if we add 1 BTU of heat to 1 lbm of water, the temperature will rise 1°F.
C. Introduction to Steam Tables
1. When a teapot of water is placed on a hot burner, sensible heat begins to heat the water. The energy added to the water raises its internal energy and its temperature. When the water reaches 212°F, the temperature no longer rises as latent heat begins to change the water from a liquid to a vapor. The mass inside the teapot is slowly changing from a 100% water / 0% steam mixture into a 0% water / 100% steam mixture. If we add only half the necessary latent heat, then only half the water will boil into steam. The result would be a 50% water / 50% steam mixture at 212°F. If we add all the latent heat necessary, then the water at 212°F changes completely into steam at 212°F. Continuing to add heat to the 212°F steam results in a temperature increase (superheating), and we are again raising the temperature by adding sensible heat. Refer to figure 3.2-1 (sensible/latent heat and enthalpy).
2. While the properties of water at atmospheric pressure are commonly known, water under different pressures will exhibit different properties. When water is boiled at pressures higher than atmospheric, the same events occur as described above with two exceptions. First, the boiling temperature will be higher than 212°F. Second, less latent heat is required to be added to change the water completely into steam. If water were to be boiled at a pressure lower than atmospheric pressure, then we would find that the boiling temperature would be less than 212°F and a larger amount of latent heat would be required to change the water completely into steam. Refer to figure 3.2-2 (temperature vs. latent heat).
a. When water is below the boiling point, the addition of heat is seen as sensible heat. This water is said to be a subcooled liquid. When enough sensible heat is added so that the temperature of the water approaches saturation temperature but no steam has yet been formed, the water is said to be a saturated liquid.
b. As the water is transformed from a saturated liquid to saturated steam, boiling is occurring. As latent heat is added, the temperature of the water remains the same but the saturated liquid is being changed into a saturated vapor. During this period the water is referred to as a liquid/vapor mixture. When enough latent heat is added so that all of the liquid is converted into vapor, the water becomes a saturated vapor. Note that the saturated vapor is 100% vapor and exists at the same temperature as the saturated liquid. Above the saturated steam point, vapor exists at a temperature higher than saturation temperature. This is the superheated vapor region.
c. Steam tables are a useful tool for determining the properties of steam and water at various temperatures and pressures. The steam tables are broken into three tables.
D. Mollier Diagram
1. The Mollier diagram is a small portion of data from the steam tables graphed onto enthalpy-entropy coordinates. It presents the region that is commonly found in propulsion plant steam systems. Examine the last section of the steam tables for a representation of a Mollier diagram.
2. Locating information off the Mollier diagram is done as follows: The horizontal axis is entropy (s) in BTU/lbm-°R. The vertical axis is enthalpy (h) in BTU/lbm. The dark line across the middle of the chart is called a “steam dome” because of its shape. Above this line, the data is for superheated steam. Below this line, the data is for a steam-water mixture. The data directly on the line is for saturated steam.
3. To find data in the steam-water mixture region of the chart, enter the chart using the absolute pressure and %-moisture (y). Once you find the intersection of these two parameters, read off the number directly across from the intersection point for enthalpy. Read off the number directly below the intersection point for entropy.
To find data in the superheated region of the chart, enter the chart using the measured temperature and pressure of the steam. Again, find the intersection point of these two parameters and read off the values for entropy and enthalpy. Notice that moisture does not plot in the superheat region. This is because moisture is a parameter which only exists in saturated conditions.
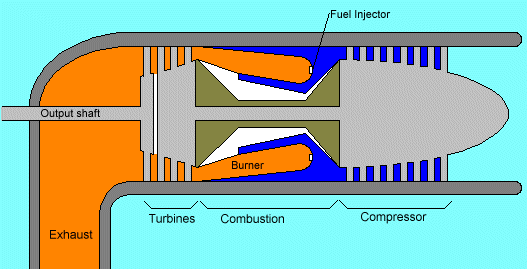
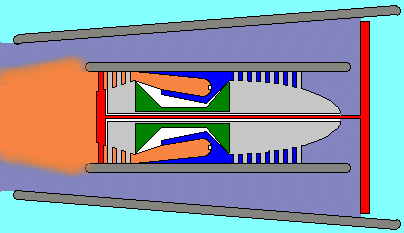




 However there are variations...
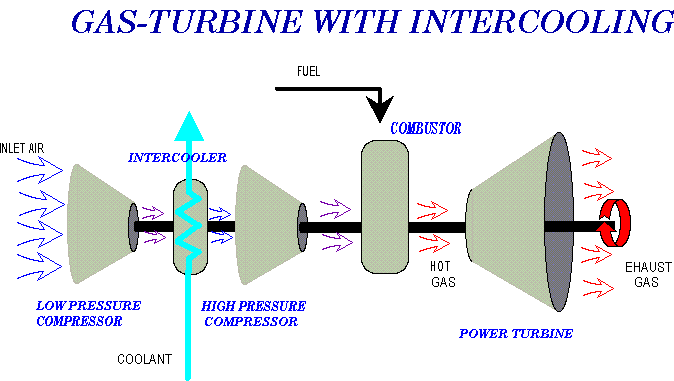
However there are variations...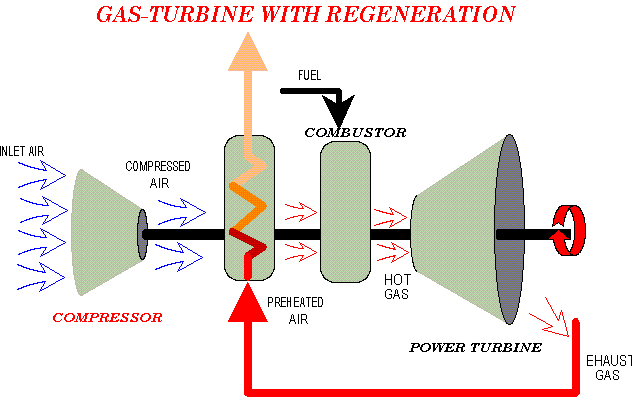
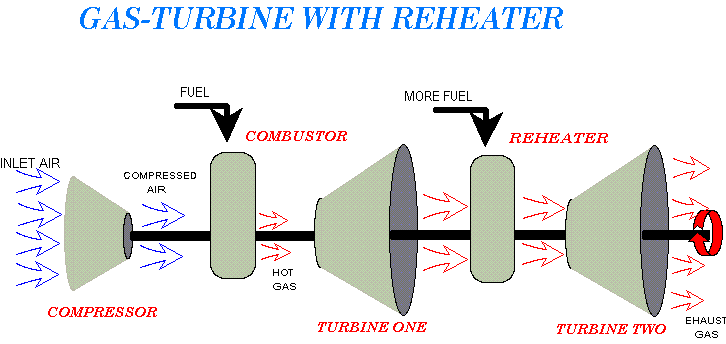
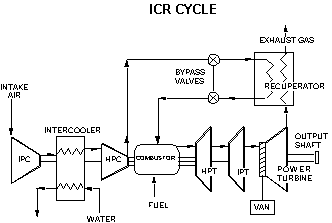
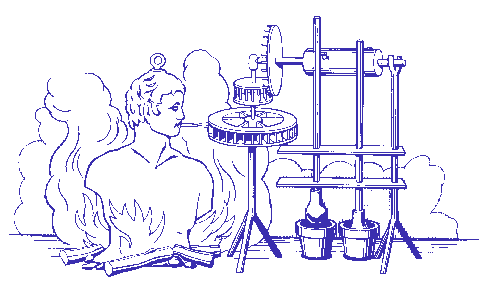
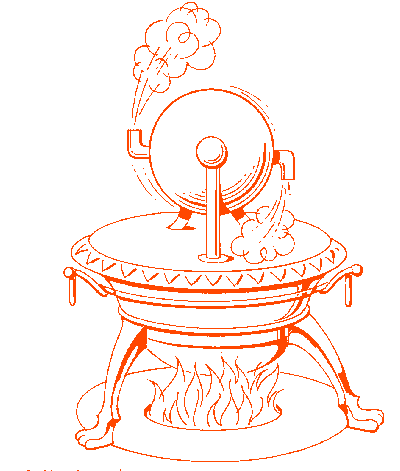 The earliest example of jet propulsion can be traced as far back as 150 BC to an Egyptian named Hero. Hero invented a toy that rotated on top of a boiling pot due to the reaction effect of hot air or steam exiting several nozzles arranged radially around a wheel. He called this invention an aeolipile.
The earliest example of jet propulsion can be traced as far back as 150 BC to an Egyptian named Hero. Hero invented a toy that rotated on top of a boiling pot due to the reaction effect of hot air or steam exiting several nozzles arranged radially around a wheel. He called this invention an aeolipile.