Description of the Carnot Cycle 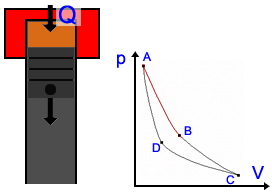 Stage 1: In the first stage, the piston moves downward while the engine absorbs heat from a source and gas begins to expand. The portion of the graphic from point A to point B represents this behavior. Because the temperature of the gas does not change, this kind of expansion is called isothermic. Stage 1: In the first stage, the piston moves downward while the engine absorbs heat from a source and gas begins to expand. The portion of the graphic from point A to point B represents this behavior. Because the temperature of the gas does not change, this kind of expansion is called isothermic.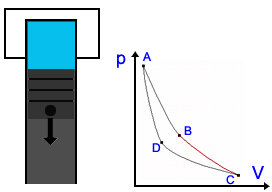 Stage 2: In the second stage, the heat source is removed; the piston continues to move downward and the gas is still expanding while cooling (lowering in temperature). It is presented by the graphic from point B to point C. This stage is called a adiabatic expansion (Energy stays) Stage 2: In the second stage, the heat source is removed; the piston continues to move downward and the gas is still expanding while cooling (lowering in temperature). It is presented by the graphic from point B to point C. This stage is called a adiabatic expansion (Energy stays)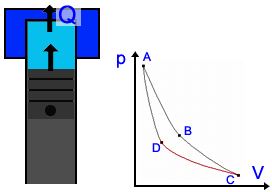 Stage 3: The piston begins to move upward and the cool gas is recompressed in the third stage. The heat goes to sink. Point C point D represents the decrease in volume and increase in pressure. The engine gives energy to the environment. This stage is called isothermal compression. Stage 3: The piston begins to move upward and the cool gas is recompressed in the third stage. The heat goes to sink. Point C point D represents the decrease in volume and increase in pressure. The engine gives energy to the environment. This stage is called isothermal compression.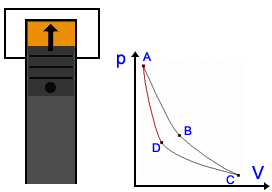 Stage 4: In the final stage, the piston to move upward and the cool gas is secluded and compressed. Its temperature rises to its original state. Point C to point D illustrate this behavior; a continuing increase in pressure and decrease in volume to their initial position. Energy stays, so it's an adiabatic compression. Stage 4: In the final stage, the piston to move upward and the cool gas is secluded and compressed. Its temperature rises to its original state. Point C to point D illustrate this behavior; a continuing increase in pressure and decrease in volume to their initial position. Energy stays, so it's an adiabatic compression. Application in engines Application in enginesThe Carnot Cycle forms the perfect process of a heat engine. Many engineers tried to reach this kind of cycle. Rudolf Diesel had the most success and his engine is nearly as perfect as the Carnot engine (See Technology part). There are also other cycles, e.g. the Stirling Cycle you see on the right side. |
Wednesday, July 14, 2010
The Carnot Cycle
Subscribe to:
Post Comments (Atom)





0 comments:
Post a Comment